Image Credit – Hema
Carbohydrates are essential biomolecules that serve as the primary source of energy in living organisms. To study these sugars, biochemists rely on a variety of laboratory tests. One important and classical method is the osazone test detection reducing sugar, developed by the German chemist Emil Fischer. This test is widely used in laboratories and classrooms to detect the presence of reducing sugars and to differentiate among common monosaccharides and disaccharides. In this article, we will explore the principle, procedure, applications, and significance of the osazone test detection reducing sugar.
Osazone Test Detection Reducing Sugar: A Perfect Guide
What is the Osazone Test?
The osazone test detection reducing sugar is a carbohydrate test in which reducing sugars react with phenylhydrazine to form distinctive crystalline compounds called osazones. These osazones have unique shapes depending on the type of sugar present. By observing these crystals under a microscope, scientists can confirm the presence of reducing sugars and identify their type.
Reducing sugars such as glucose, fructose, maltose, galactose, and lactose are typically tested using this method. Since these sugars possess free aldehyde or ketone groups, they readily undergo reactions with phenylhydrazine to form osazones.
Principle of the Osazone Test
The test is based on the chemical reaction between the carbonyl group of a reducing sugar and phenylhydrazine. Here’s how it works:
- Reducing sugars have a free aldehyde (-CHO) or ketone (C=O) group.
- When phenylhydrazine is added to the sugar solution and heated, the carbonyl group reacts to form phenylhydrazones.
- Further reaction at the carbon atoms next to the carbonyl group leads to the formation of osazones.
- These osazones appear as yellow crystalline precipitates with unique shapes that can be identified microscopically.
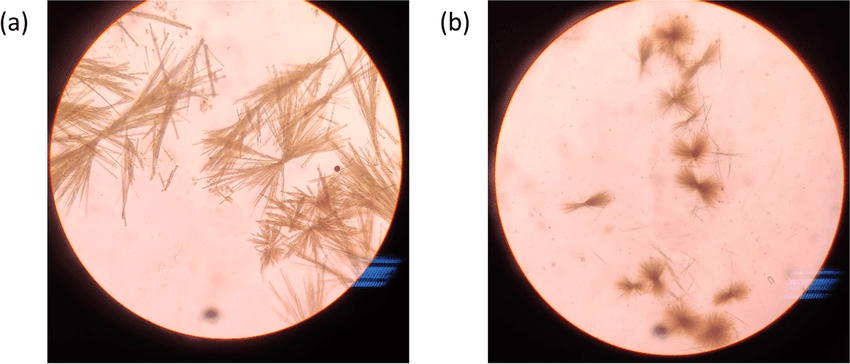
Different sugars produce different osazone crystals:
- Glucose → Needle-shaped crystals
- Fructose → Needle-shaped crystals (similar to glucose)
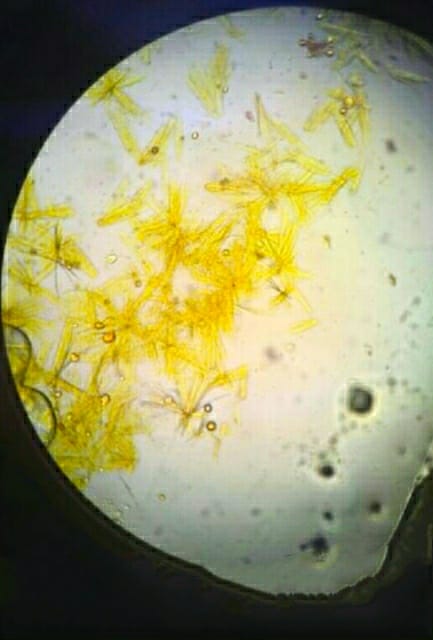
- Maltose → Sunflower-like crystals
- Lactose → Powder puff or cotton wool-shaped crystals
Thus, the osazone test detection reducing sugar not only confirms the presence of reducing sugars but also helps distinguish between them.
Procedure of the Osazone Test
Here is the step-by-step method to perform the Osazone Test for detecting reducing sugars:
- Take about 5 ml of sugar solution in a clean test tube.
- Add 0.5 g of phenylhydrazine hydrochloride.
- Add 0.8 g of sodium acetate and 1 ml of acetic acid.
- Mix the solution well and heat it in a boiling water bath for 30 minutes.
- Allow the mixture to cool and observe the precipitated yellow osazone crystals under a microscope.
Applications of the Osazone Test
- Detection of Reducing Sugars: Helps confirm the presence of monosaccharides like glucose and fructose.
- Differentiation of Sugars: By identifying the unique crystalline forms, the test helps distinguish between sugars such as lactose, maltose, and mannose.
- Educational Tool: Commonly used in teaching biochemistry to demonstrate the behavior of reducing sugars.
- Clinical Importance: Historically used in the diagnosis of diabetes mellitus by detecting glucose in urine samples.
Limitations of the Osazone Test Detection Reducing Sugar
While useful, the Osazone Test also has some drawbacks:
- It cannot clearly differentiate between glucose and fructose since both form needle-shaped crystals.
- It requires heating and handling of phenylhydrazine, which is a toxic chemical.
- Modern techniques like chromatography and spectrophotometry have largely replaced this test in clinical labs.
The Osazone Test detection reducing sugars is a remarkable example of how simple chemical reactions can be used to analyze biomolecules. Though largely replaced by advanced analytical techniques today, it remains an important experiment in biochemistry education. By forming unique crystalline osazones, this test demonstrates how chemistry can visually reveal the presence and type of sugars.
Whether in a teaching lab or as part of biochemical history, the Osazone Test continues to be a fascinating method for understanding carbohydrates.
Frequently Asked Questions
What is the principle of the osazone test detection reducing sugar?
The principle of the osazone test detection reducing sugar lies in the reaction of the free aldehyde or ketone group of reducing sugars with phenylhydrazine. On heating, this reaction forms yellow crystalline compounds known as osazones. Each sugar produces crystals of unique shapes, helping in both detection and differentiation of sugars.
Which sugars can be identified by the osazone test detection reducing sugar?
The osazone test detection reducing sugar is commonly used to identify glucose, fructose, maltose, galactose, and lactose. For example, glucose and fructose form needle-shaped crystals, maltose forms sunflower-shaped crystals, and lactose forms cotton wool-like crystals. These crystal patterns help distinguish one sugar from another.
Why is the osazone test detection reducing sugar important in biochemistry?
The osazone test detection reducing sugar is important because it provides a simple, visual method to confirm the presence of reducing sugars. Although modern techniques like chromatography and spectrophotometry have replaced it in advanced labs, the test remains an excellent educational tool in biochemistry, helping students understand carbohydrate chemistry and reactions.

